The chemistry of whale oil
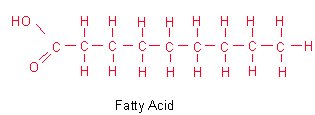
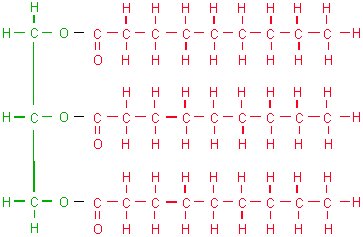
Glycerol contains 3 carbons and 3 hydroxyl groups which can react with 3 fatty acids to form a triglyceride or neutral fat molecule. These triglycerides are the major component of whale oil. Many commercial uses of whale oil, for example soap, margarine and explosives manufacture, involves breaking down the oil to give glycerol and fatty acids.
 |
 |
 |
|
